|
Current
Research |
|
1. Role of Estradiol in the Control of
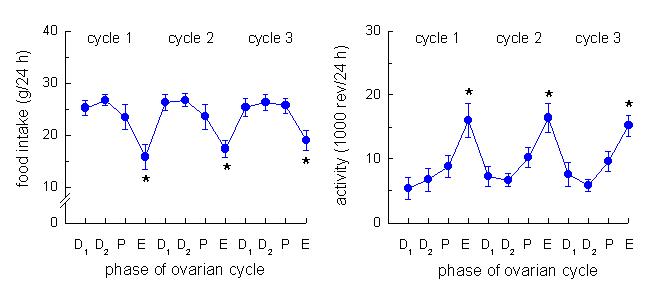
Food Intake A distinctive pattern of food intake and running
wheel activity are apparent in female rats across the 4-day estrous cycle.
During estrus, just after the peak in estradiol secretion, food intake is
decreased 20-40% and running wheel activity is increased by over 100%.
Both of these effects are abolished by surgical removal of the ovaries but
they can be reinstated by estradiol replacement alone. Thus, the behavioral
changes associated with estrus are mediated by estradiol. The following figure from our lab illustrates
the estrous-related changes in food intake and running wheel activity in a
group of female rats. Abbreviations: D1; diestrus 1, D2; diestrus 2, P;
proestrus, E; estrus. |
|
In our lab we are using multiple
approaches to investigate the mechanism by which estradiol controls food
intake in female rats. Behavioral and pharmacological studies are
designed to investigate the effects of estradiol and other satiety peptides
on spontaneous feeding and locomotor activity in female rats. Immunohistochemical
studies focus on the effects of estradiol on neuronal activity and
peptide expression within brain regions that process feeding-stimulated
signals. For example, using the induction of c-Fos-like immunoreactivity as a
marker of neuronal activation, we have recently discovered that estradiol
treatment in ovariectomized rats increases neuronal activity induced by
feeding-related stimuli in multiple brain regions. Thus, estradiol may
decrease food intake during estrus by increasing the central processing of
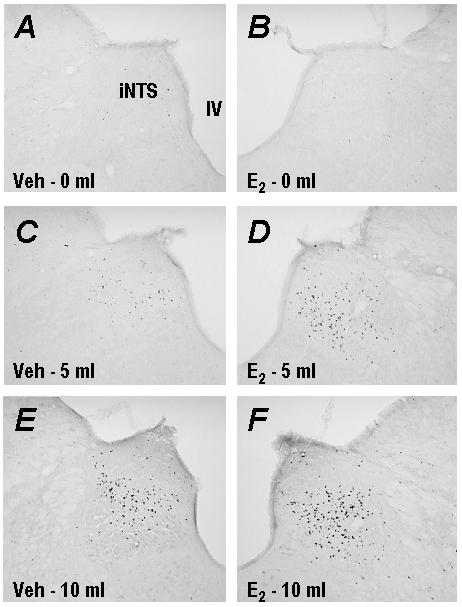
peripherally generated satiety signals. The following illustration includes
representative photomicrographs of feeding-stimulated c-Fos-like
immunoreactivity in the nucleus of the solitary tract, a brain region
implicated in the physiological control of food intake. Estradiol treatment
significantly enhanced the amount of c-Fos expression (compare right panels
with left panels) induced by 5 and 10 ml of milk consumption. Abbreviations:
iNTS; intermediate nucleus of the solitary tract, IV; fourth ventricle, Veh;
vehicle-treated rat, E2; estradiol-treated rat. |
|
Finally, molecular studies
focus on estradiol-mediated changes in gene expression. Currently, we are
using in situ hybridization techniques to map the distribution of
estrogen receptors in hindbrain regions involved in the physiological control
of food intake. Future experiments will use expression profiling techniques
to identify the phenotype of neurons that are regulated by estradiol. |
|
2. Activity-Based Anorexia in Female
Rats. Anorexia nervosa is a complex eating disorder characterized in part by
hypophagia, disorganized eating patterns, body weight loss, hyperactivity,
and a dysregulation of the hypothalamic-pituitary-gonadal axis. About 90% of
clinically-diagnosed cases involve women. Therapeutic treatment of the
symptoms of anorexia nervosa is limited due to our lack of understanding of
the multiple factors that underlie this complex disorder. Animal studies have shown that female rats
maintained on a food restriction schedule and given free access to running
wheels display hypophagia, rapid body weight loss, increased running wheel
activity, and a disruption of the estrous cycle. This phenomenon has been
labeled activity-based anorexia. In our lab, we are using this animal model to
investigate the physiological and neural mechanisms underlying anorexia
nervosa. Currently, there are several ongoing projects. 1) We are characterizing the behavioral
profile of rats with activity-based anorexia by monitoring
spontaneous feeding and activity patterns in rats prior to, during, and after
the induction of activity-based anorexia. These studies are designed to screen
for behavioral characteristics that may increase susceptibility to developing
an eating disorder. 2) Because anorexia nervosa is more prevalent in
women than in in men, we are examining the involvement of estradiol in
the development and maintenance of activity-based anorexia. 3) To investigate the neural mechanism
underlying activity-based anorexia, we are examining the patterns of
feeding-induced neuronal activation in rats with and without activity-based
anorexia. |